1.4 Can Matter Change its State?
NCERT Class 9 Science Textbook for Blind and Visually Impaired Students made Screen Readable by Professor T K Bansal.
We all know from our observation that water can exist in three states of matter-
• solid, as ice,
• liquid, as the familiar water, and
• gas, as water vapours.
What happens inside the matter during this change of state?
What happens to the particles of matter during the change of states?
How does this change of state take place?
We need answers to these questions, isn’t it?
1.4.1 EFFECT OF CHANGE OF TEMPERATURE
Activity 1.12
- Take about 150 g of ice in a beaker and suspend a laboratory thermometer so that its bulb is in contact with the ice, as in Figure 1.6.
- Figure 1.6: (a) Conversion of ice to water, (b) conversion of water to water vapour
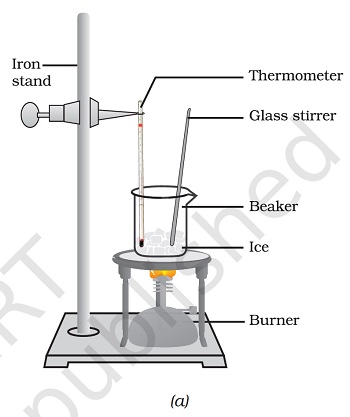
“Image description by Dr T K Bansal begins: This figure is in two parts (a) and (b). Part (a) shows a beaker containing ice, a stirrer, and a thermometer for measurement of temperature. The beaker is placed on a tripod stand and is heated from below with the help of a burner.
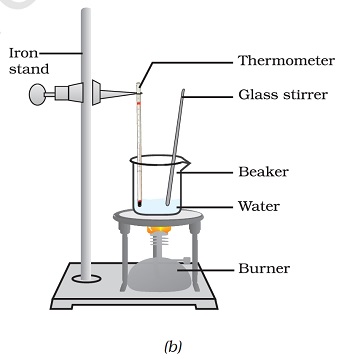
Part (b) The arrangement in this part is exactly the same as that of part (a), with the only difference that the beaker contains water instead of ice. Image description ends.”
- Start heating the beaker on a low flame.
- Note the temperature when the ice starts melting.
- Note the temperature when all the ice has converted into water.
- Record your observations for this conversion of solid to liquid state.
- Now, put a glass rod in the beaker and heat while stirring till the water starts boiling.
- Keep a careful eye on the thermometer reading till most of the water has vaporised.
- Record your observations for the conversion of water in the liquid state to the gaseous state.
On increasing the temperature of solids, the kinetic energy of the particles increases. Due to the increase in kinetic energy, the particles start vibrating with greater speed. The energy supplied by heat overcomes the forces of attraction between the particles. The particles leave their fixed positions and start moving more freely. A stage is reached when the solid melts and is converted to a liquid. The temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
The melting point of a solid is an indication of the strength of the force of attraction between its particles.
The melting point of ice is 273.16 K*.
Start of yellow box:
[ *Note: Kelvin is the SI unit of temperature, 0 degree C =273.16 K. For convenience, we take 0 degree C = 273 K after rounding off the decimal. To change a temperature on the Kelvin scale to the Celsius scale you have to subtract 273 from the given temperature, and to convert a temperature on the Celsius scale to the Kelvin scale you have to add 273 to the given temperature.] End of yellow box.
The process of melting, that is, change of solid state into liquid state is also known as fusion. When a solid melts, its temperature remains the same, so where does the heat energy go?
You must have observed, during the experiment of melting, that the temperature of the system does not change after the melting point is reached, till all the ice melts. This happens even though we continue to heat the beaker, that is, we continue to supply heat. This heat gets used up in changing the state by overcoming the forces of attraction between the particles. As this heat energy is absorbed by ice without showing any rise in temperature, it is considered that it gets hidden into the contents of the beaker and is known as the latent heat. The word latent means hidden. The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion. So, particles in water at 0 degree C (273 K) have more energy as compared to particles in ice at the same temperature.
When we supply heat energy to water, particles start moving even faster. At a certain temperature, a point is reached when the particles have enough energy to break free from the forces of attraction of each other. At this temperature the liquid starts changing into gas. The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point. Boiling is a bulk phenomenon. Particles from the bulk of the liquid gain enough energy to change into the vapour state.
For water this temperature is 373 K (100 degree C = 273 + 100 = 373 K).
Can you define the latent heat of vaporisation? Do it in the same way as we have defined the latent heat of fusion. Particles in steam, that is, water vapour at 373 K (100 ℃) have more energy than water at the same temperature. This is because particles in steam have absorbed extra energy in the form of latent heat of vaporisation.
Solid on heating gives Liquid, and liquid on heating gives Gas.
Gas on cooling gives Liquid, and liquid on cooling gives Solid.
So, we infer that the state of matter can be changed into another state by changing the temperature.
We have learnt that substances around us change state from solid to liquid and from liquid to gas on application of heat. But there are some materials that change directly from solid state to the gaseous state and vice versa without changing into the liquid state.
Activity 1.13
- Take some camphor or ammonium chloride. Crush it and put it in a china dish.
- Put an inverted funnel over the china dish.
- Put a cotton plug on the stem of the funnel, as shown in Figure 1.7.
- Figure 1.7: Sublimation of ammonium chloride
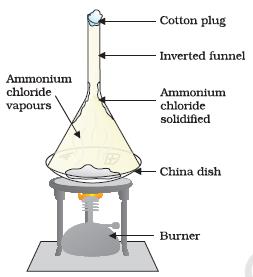
“Image Description by Dr T K Bansal starts: The figure shows a china dish having some solid ammonium chloride in it. The china dish is placed on a tripod stand and is heated from below with the help of a burner. An inverted funnel with its stem closed with the help of a cotton plug is placed on the china dish. It is shown that the vapours of ammonium chloride are rising from the china dish and are getting condensed on the inner surface of the inverted funnel. Image description ends.”
- Now, heat slowly and observe.
- What do you infer from the above activity?
A change of state directly from solid to gas without changing into liquid state (or vice versa) is called sublimation.
Start of yellow box: [ * atmosphere (atm) is a unit of measuring pressure exerted by a gas. The unit of pressure is Pascal (Pa read as P a): 1 atmosphere = 1.01*10^5 Pa. The pressure of air in atmosphere is called atmospheric pressure. The atmospheric pressure at sea level is 1 atmosphere, and is taken as the normal atmospheric pressure.] End of yellow box.
1.4.2 EFFECT OF CHANGE OF PRESSURE
We have already learnt that the difference in various states of matter is due to the difference in the distances between the constituent particles. What will happen when we start putting pressure and compress a gas enclosed in a cylinder? Will the particles come closer? Do you think that increasing or decreasing the pressure can change the state of matter?
Figure 1.8: By applying pressure, particles of matter can be brought close together.
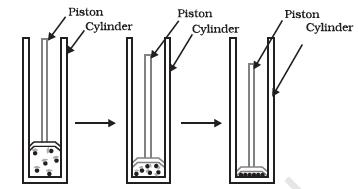
“Image description by Dr T K Bansal begins: The figure is in three parts. Part 1 shows a cylinder closed at the bottom and has a moveable piston fitted in it. Initially the piston has a large volume under it and contains some gas in it. It is shown that the molecules of the gas are farther apart from each other. In Part 2 the piston is moved inwards thereby decreasing the volume of the gas. The molecules of the gas moves closer to each other. In the third part, the piston is moved further down, thereby compressing the gas further. The gas molecules move so close to each other that the gas is converted into a liquid. Image description ends.
Applying pressure and reducing temperature can liquefy gases. Have you heard of solid carbon dioxide ( C O2 )? It is stored under high pressure. Solid C O2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere* without coming into liquid state. This is the reason that solid carbon dioxide is also known as dry ice.
Thus, we can say that pressure and temperature determine the state of a substance, whether it will be solid, liquid or gas.
Figure 1.9: Interconversion of the three states of matter
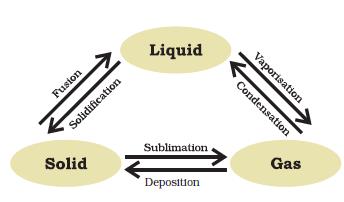
“Image Description by Dr T K Bansal begins: This figure is in the form of a flow diagram in which the three states of matter are mentioned and how does one state gets converted into another, that is: Solid on sublimation gives gas; Gas on deposition gives Solid; Solid on fusion (melting) gives Liquid; Liquid on solidification gives Solid; Liquid on evaporation gives gas; and Gas on condensation gives Liquid. Image description ends.”
Questions
Q1. Convert the following temperature to celsius scale:
(a). 300 K
(b). 573 K
A1
(a)27 C,
(b) 200 C
Q2. What is the physical state of water at:
(a). 250°C
(b). 100°C ?
A2.
(A). Vapour, and
(b). boiling.
Q3. For any substance, why does the temperature remain constant during the change of state?
A3. As the heat which should have been used for changing the temperature of the substance is now used to overcome the intermolecular force of attraction between the particles of matter in order to change its state. The temperature of the substance remains constant.
Q4. Suggest a method to liquefy atmospheric gases.
A4. By cooling and applying high pressure.
