1.3 States of Matter
NCERT Class 9 Science Textbook for Blind and Visually Impaired Students made Screen Readable by Professor T K Bansal.
Observe different types of matter around you. What are its different states? We can see that matter around us exists in three different states- solid, liquid and gas. These states of matter arise due to the variation in the characteristics of the particles of matter.
Now, let us study about the properties of these three states of matter in detail.
1.3.1 THE SOLID STATE
Activity 1.9
- Collect the following articles- a pen, a book, a needle and a piece of thread.
- Sketch the shape of the above articles in your notebook by moving a pencil around them.
- Do all these have a definite shape, distinct boundaries and a fixed volume?
- What happens if they are hammered, pulled or dropped?
- Are these capable of diffusing into each other?
- Try compressing them by applying force. Are you able to compress them?
All the above are examples of solids. We can observe that all these have a definite shape, distinct boundaries and fixed volumes, that is, have negligible compressibility. Solids have a tendency to maintain their shape when subjected to outside force. Solids may break under force but it is difficult to change their shape, so they are rigid.
Consider the following:
(a) What about a rubber band, can it change its shape on stretching? Is it a solid?
(b) What about sugar and salt? When kept in different jars these take the shape of the jar. Are they solid?
(c) What about a sponge? It is a solid yet we are able to compress it. Why?
All the above are solids as:
• A rubber band changes shape under force and regains the same shape when the force is removed. If excessive force is applied, it breaks.
• The shape of each individual sugar or salt crystal remains fixed, whether we take it in our hand, put it in a plate or in a jar.
• A sponge has minute holes, in which air is trapped, when we press it, the air is expelled out and we are able to compress it.
1.3.2 THE LIQUID STATE
Activity 1.10
- Collect the following:
- (a) water, cooking oil, milk, juice, a cold drink.
(b) containers of different shapes. Put a 50 mL mark on these containers using a measuring cylinder from the laboratory. - What will happen if these liquids are spilt on the floor?
- Measure 50 mL of any one liquid and transfer it into different containers one by one. Does the volume remain the same?
- Does the shape of the liquid remain the same ?
- When you pour the liquid from one container into another, does it flow easily?
We observe that liquids have no fixed shape but have a fixed volume. They take up the shape of the container in which they are kept. Liquids flow and change shape, so they are not rigid but can be called fluid.
Refer to Activities 1.4 and 1.5 where we saw that solids and liquids can diffuse into liquids. The gases from the atmosphere diffuse and dissolve in water. These gases, especially oxygen and carbon dioxide, are essential for the survival of aquatic animals and plants.
All living creatures need to breathe for survival. The aquatic animals can breathe under water due to the presence of dissolved oxygen in water. Thus, we may conclude that solids, liquids and gases can diffuse into liquids. The rate of diffusion of liquids is higher than that of solids. This is due to the fact that in the liquid state, particles move freely and have greater space between each other as compared to particles in the solid state.
1.3.3 THE GASEOUS STATE
Have you ever observed a balloon seller filling a large number of balloons from a single cylinder of gas? Enquire from him how many balloons is he able to fill from one cylinder. Ask him which gas does he have in the cylinder.
Activity 1.11
- Take three 100 mL syringes and close their nozzles by rubber corks, as shown in Figure1.4.
Figure 1.4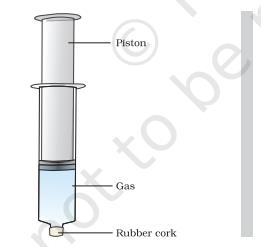
- Remove the pistons from all the syringes.
- Leaving one syringe untouched, fill water in the second and pieces of chalk in the third.
- Insert the pistons back into the syringes. You may apply some Vaseline on the pistons before inserting them into the syringes for their smooth movement.
- Now, try to compress the content by pushing the piston in each syringe.
- What do you observe? In which case was the piston easily pushed in?
- What do you infer from your observations?
We have observed that gases are highly compressible as compared to solids and liquids. The liquefied petroleum gas (LPG) cylinder that we get in our home for cooking or the oxygen supplied to hospitals in cylinders is compressed gas. Compressed natural gas (CNG) is used as fuel these days in vehicles. Due to its high compressibility, large volumes of a gas can be compressed into a small cylinder and transported easily.
We come to know of what is being cooked in the kitchen without even entering there, by the smell that reaches our nostrils. How does this smell reach us? The particles of the aroma of food mix with the particles of air spread from the kitchen, reach us and even farther away. The smell of hot cooked food reaches us in seconds; compare this with the rate of diffusion of solids and liquids. Due to high speed of particles and large space between them, gases show the property of diffusing very fast into other gases.
In the gaseous state, the particles move about randomly at high speed. Due to this random movement, the particles hit each other and also the walls of the container. The pressure exerted by the gas is because of this force exerted by gas particles per unit area on the walls of the container.
Figure1.5: a, b and c show the magnified schematic piture of the three states of matter. The motion of the particles can be seen and compared in the three staes of matter.
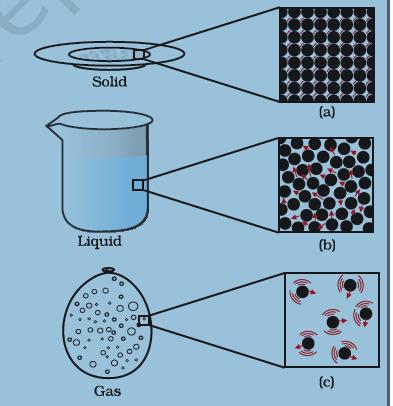
“Image Description by Dr T K Bansal begins: This figure consists of three parts. Part (a) depicts a solid plate. The magnified image of the plate shows that the constituent particles of the plate (atoms) are touching each other and are at rest. Part (b) depicts a beaker containing a liquid. The magnified picture of the liquid shows that the particles are close to each other, but relatively farther away as compared to that in the solid as shown in Part (a). It also shows that the particles are in motion. Part (c) shows a balloon filled with a gas. The magnified picture of the gas shows that the particles are very far away from each other. Only a few particles are visible in the magnified picture. It is also clear that the particles are moving rapidly in a gas. Image description Ends.”
Questions
Q1. The mass per unit volume of a substance is called density.
(density = mass/volume).
Arrange the following in order of increasing density -
air, exhaust from chimneys, honey, water, chalk, cotton and iron.
A1. air, exhaust from chimneys, cotton, water, honey, chalk and iron.
Q2.
(a) Tabulate the differences in the characteristics of states of matter.
(b) Comment upon the following:
rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Q3. Give reasons
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
A3.
(a) This is because the particles of matter in gases are very loosely packed and they move about randomly. Due to this random movement, they spread wide apart.
(b) Since, particles of gases move about randomly, they hit each other and also against the walls of the container due to this random movement. They exert pressure on the walls of the container.
(c) Since a wooden table has a fixed shape, fixed volume, and since it is negligible to compress and is rigid. It is called a solid.
(d) As we know that particles of matter have spaces between them. The particles of air have large spaces between them. In contrast, the particles of a solid like a wooden block are very tightly packed. So, we are able to move our hand in air because our hand occupies those spaces that are left by the particles of air. But, as there is no such gap in solids we are not able to do so.
Q4. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
A4. As compared to all other substances, ice has a lower density as compared to liquid water. It is due to this reason ice floats on water.
