1.1 Physical Nature of Matter
NCERT Class 9 Science Textbook for Blind and Visually Impaired Students made Screen Readable by Professor T K Bansal.
1.1.1 MATTER IS MADE UP OF PARTICLES
For a long time, two schools of thought prevailed regarding the nature of matter.
One school believed matter to be continuous like a block of wood,
whereas, the other thought that matter was made up of particles like sand.
Let us perform an activity to decide about the nature of matter - is it continuous or particulate?
Activity 1.1
- Take a 100 mL beaker.
- Fill half the beaker with water and mark the level of water.
- Dissolve some salt/ sugar with the help of a glass rod.
- Observe any change in water level.
- What do you think has happened to the salt?
- Where does it disappear?
- Does the level of water change?
In order to answer these questions we need to use the idea that matter is made up of particles. What was there in the spoon, salt or sugar, has now spread throughout water. This is illustrated in Figure 1.1.
Figure 1.1 : when we dissolve salt in water, the particles of salt get into the spaces between particles of water
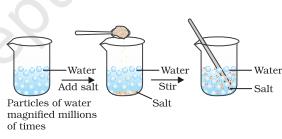
“Image Description by Dr. T K Bansal begins: The figure has three parts; Part 1 depicts a beaker half filled with clean water. Part 2 depicts that salt is being poured into the water contained in the beaker with the help of a spoon. It is shown that the salt added to the water has satteled at the bottom of the beaker. Part 3 depicts that the water containing salt is stirred with the help of a glass rod, and the salt has completely dissolved in the water to give you the clear solution. Image description ends”
1.1.2 HOW SMALL ARE THESE PARTICLES OF MATTER?
Activity 1.2
- Take 2-3 crystals of potassium permanganate and dissolve them in 100 mL of water.
- Take out approximately 10 mL of this solution and put it into 90 mL of clear water.
- Take out 10 mL of this solution and put it into another 90 mL of clear water.
- Keep diluting the solution like this 5 to 8 times.
- Is the water still coloured ?
With every dilution, though the colour becomes light, it is still visible.
Figure 1.2: Estimating how small are the particles of matter.
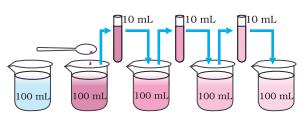
“Image description by Dr T K Bansal begins: This figure has five parts; Part 1 shows a beaker in which 100 ml of water is added. A few crystals of potassium permanganate are added to it and the water in the beaker attains a dark pink color. Part 2 depicts that 10 ml of solution from the first beaker is shifted to 90 ml of pure water in another beaker. The color of the solution is pink but is lighter than that in the previous solution. Parts 3, 4 and 5 are the repetition of the part 2. It can be seen that on every dilution the color of the solution though pink, is lighter than the previous solution. Image description ends.”
This experiment shows that just a few crystals of potassium permanganate can colour a large volume of water (about 1000 L). So we conclude that there must be millions of tiny particles in just one crystal of potassium permanganate, which keep on dividing themselves into smaller and smaller particles. Ultimately a stage is reached when the particles cannot divide further into smaller particles.
The same activity can be done using 2 mL of antiseptic liquid 'Dettol' instead of potassium permanganate. The smell of Dettol can be detected even on repeated dilution.
Important note
The particles of matter are very small - they are small beyond our imagination!!!!
